The four companies, Benjamin Moore & Co., Inc., ICP Construction Inc., YOLO Colorhouse, LLC, and Imperial Paints, LLC, agreed to settle Federal Trade Commission (FTC) allegations that they promoted products as emission-free or containing zero volatile organic compounds (VOCs) during and immediately after painting without having adequate substantiation for making those claims. Some advertisements from the companies also made explicit unsubstantiated safety claims regarding babies, children, pregnant women, and other sensitive populations, such as those suffering from asthma or allergies. The FTC is now taking comment on the Consent Orders for the four companies.
The FTC published Green Guides, which are designed to help marketers ensure that their environmental benefit claims are truthful and non-deceptive in accordance with Section 5 of the FTC Act, 15 U.S.C. §45. The companies failed to meet the FTC’s Green Guides and the FTC’s Enforcement Policy on Zero-VOC claims, and did so at their own peril. The Orders follow the Green Guides and the Enforcement Policy in that the companies cannot make unqualified zero-emission or zero-VOC claims unless the emissions and VOC content is actually zero (which is a difficult standard to meet), or the companies can meet the FTC’s de minimis standard, i.e., emissions and VOC content can be at trace levels.
The FTC’s Enforcement Policy on Zero-VOC claims describes the “trace level” test a company must meet in order to make unqualified “zero” or “free-of” VOC claims: (1) VOCs have not been intentionally added to the product; (2) the presence of VOCs at that level does not cause material harm that consumers typically associate with VOCs, including but not limited to, harm to the environment or human health; and (3) the presence of VOCs at that level does not result in concentrations higher than would be found at background levels in the ambient air.
The Enforcement Policy’s “trace level” test was changed in the recent Consent Orders. In the Orders, the new “trace level” test is:
- A VOC has not been intentionally added to the covered product;
- Emission of the covered product does not cause material harm that consumers typically associate with emission, including harm to the environment or human health; and
- Emission of the covered product does not result in more than harmless concentrations of any compound higher than would be found under normal conditions in the typical residential home without interior architectural coating.
The emphasis in the “trace level” test now seems to be on “emissions,” which is defined in the Orders as any compound that is emitted or produced during application, curing, or exposure of a covered product. Additionally, it appears that the FTC further refined the third criteria pertaining to background levels by specifying where the background level measurement should be, i.e., in a typical residential home, and how the measurement should be taken, i.e., without interior architectural coating.
Based on the Consent Orders, the four companies promoted products as emission-free or zero-VOC without having adequate substantiation for making those claims. As such, they are barred from doing the following:
- Making unqualified emission-free and VOC-free claims, unless both content and emissions are actually zero, or emissions are at trace levels, beginning at application and thereafter;
- Making claims about emission, VOC levels, odor, and other environmental or health benefits, unless they are true and not misleading, and unless the companies have competent and reliable scientific evidence to back them up; and
- Providing third parties with the means of making false, unsubstantiated, or misleading representations about material facts regarding paints.
In addition, the four companies must send letters to their distributors, instructing them to stop using existing marketing materials, and provide stickers or placards to correct misleading claims appearing on product packaging or labeling in order to correct existing unsubstantiated claims. Benjamin Moore and ICP Construction must also disclose that the environmental seals appearing in their promotional materials are their own in-house designations.
It must be noted that if the FTC Commission finalizes the Orders, it plans to update the 2012 Sherwin-Williams Company and PPG Architectural Finishes Orders previously settled with the FTC over unsubstantiated zero-VOC and environmental benefit claims.

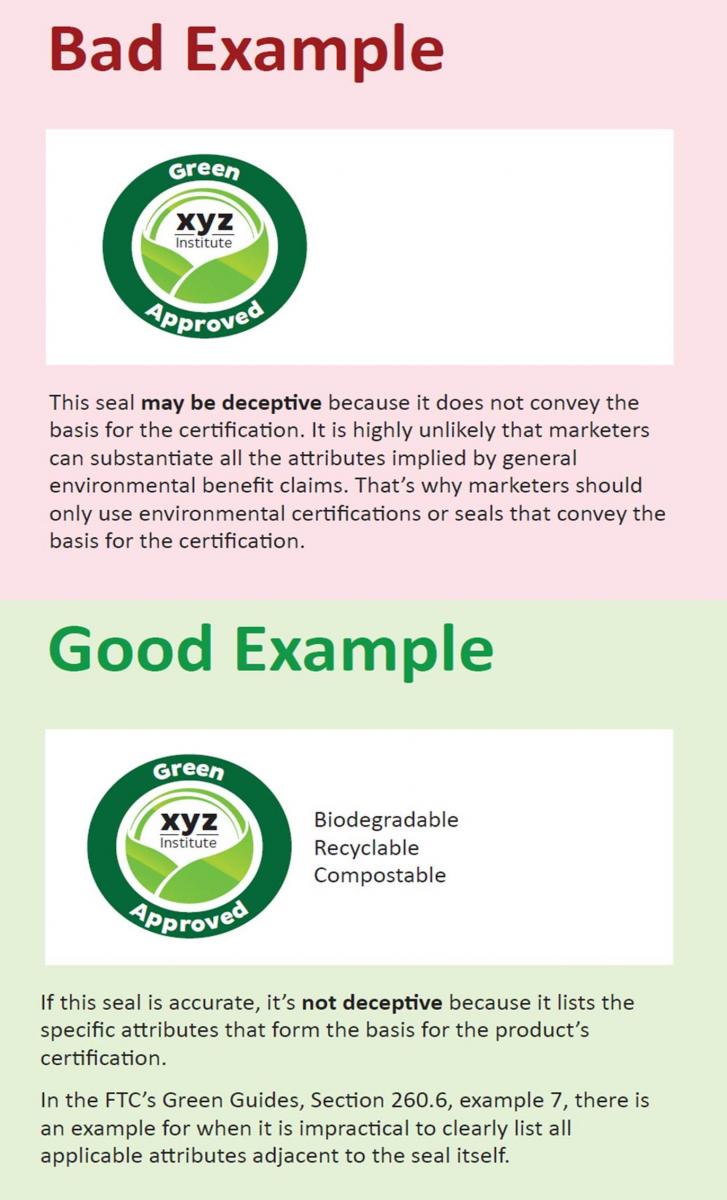 ause it is unlikely that companies can substantiate the vast array of claims that consumers can potentially interpret from an unqualified environmental certification seal, the FTC urges against using “seals that do not convey the basis for the certification.” The FTC’s blog post also includes a helpful visual illustrating good and bad examples of using an environmental certification seal (at right):
ause it is unlikely that companies can substantiate the vast array of claims that consumers can potentially interpret from an unqualified environmental certification seal, the FTC urges against using “seals that do not convey the basis for the certification.” The FTC’s blog post also includes a helpful visual illustrating good and bad examples of using an environmental certification seal (at right):